как измерить толщину мыльной плёнки? Пожалуйста подскажите формулу
Знаток
(326),
закрыт
13 лет назад
Marat
Просветленный
(25907)
13 лет назад
Толщину мыльной плёнки можно измерить, используя интерференцию света (именно из-за неё тонкие плёнки окрашены в радужные цвета) . Можно, например, направить на мыльную плёнку обычный (“белый”) луч света под углом fi, и наблюдать за цветом отражённого света. Каждый цвет соответствует определенной длине волны L (точнее, интервалу) : 600 нм (жёлтый) , 450 нм (синий) и т. д. Тогда толщину плёнки (d) Вы сможете оценить по формуле:
d = L / 4*sqr{n^2 – [sin(fi)]^2},
где n -показатель преломления мыльной воды (1.3), а sqr обозначает квадратный корень.
Источник: http:// ido.tsu.ru/ schools/ physmat/ data/ res/optika/ pract/text/ pract2.html
Задача: Какую наименьшую толщину, должна иметь мыльная плёнка, что бы отражённые лучи имели красную окраску (0,63 мкм).Белый луч падает на плёнку под углом 30 градусов.
Решение: Во первых вспоминаем волновые свойства света и такое явление как интерференция.
Соответственно, что бы в отражённом свете мы увидели красный спектр, необходимо, что бы на выходе в результате интерференции, усилились волны необходимой нам длинны (0,63 мкм).
На выходе из плёнки мы получаем два пучка света, так как у них один источник, лучи когерентны (когерентны это когда одинаковая частота и постоянная разница фаз). Нам нужно подобрать такую толщину плёнки, что бы разница хода между лучом (0,63 мкм) отражённым от поверхности плёнки и лучом который отразится от внутренней поверхности плёнки составляла величину кратную длине волны. То есть, если разница хода лучей, будет кратна длине волны, это будет условием максимума. Тогда на выходе согласно принципа суперпозиции при совпадении максимумов мы получим усиление волны нужной длины.
Задача решена.
Мне важно понимать на сколько доступно и понятно у меня получается объяснять алгоритм решения. Я работаю преподавателем и Ваши вопросы и комментарии помогут мне улучшить формат подачи материала.
Хотите больше задач? Подписывайтесь на канал, ставьте лайки, пишите комментарии. Предлагайте варианты задач, обязательно со ссылкой на источник.
На мыльную пленку нормально падает белый свет. В отраженном свете она кажется синей. При какой наименьшей толщине
пленки это возможно? Длина волны синего света в вакууме равна 480 нм. Считайте показатель преломления мыльной пленки равным показателю преломления воды.
Спрятать решение
Решение.
Разность хода для тонкой пленки определяется формулой Условие максимума
Отсюда находим толщину пленки:
Ответ: 90 нм.
Источник: Гельфгат И. М. Сборник задач по физике для 11 класса, Х.: «Гимназия», 2004 (№ 7.34)
2018-08-02
Точка мыльного пузыря, ближайшая к наблюдателю, кажется ему зеленой ($lambda = 540 нм$). Определите минимальную толщину мыльной пленки. Показатель преломления мыльной пленки $n = 1,35$.
Решение:
Интерференционные максимумы наблюдаются, когда световые лучи, отраженные от верхней и нижней поверхности пластинки, усиливают друг друга и имеют разность хода волн, равную целому числу длин волн (рис.):
$Delta l = n(AC + BC) – AD + frac{ lambda }{2} = k lambda$,
где $Delta l$ – оптическая разность хода, коэффициент п учитывает уменьшение скорости света в среде с показателем преломления $n$, а слагаемое $frac{ lambda}{2}$ возникает потому, что при отражении луча 1 от оптически более плотной среды фаза колебаний изменяется на противоположную, т. е. возникает такое же изменение фазы, как при прохождении пути $frac{ lambda}{2}$
Из соотношений $AC = BC = frac{d}{ cos beta }, AD = 2d sin alpha tg beta, n = frac{ sin alpha}{ sin beta}$ получаем
$left ( k – frac{1}{2} right ) lambda = 2d sqrt{n^{2} – sin^{2} alpha }$,
откуда
$d = frac{ left ( k – frac{1}{2} right ) lambda }{2 sqrt{n^{2} – sin^{2} alpha } }$.
Минимальная толщина пленки будет при $k = 1$ и $alpha = 0^{ circ}$.
$d = 10^{-7} м$.
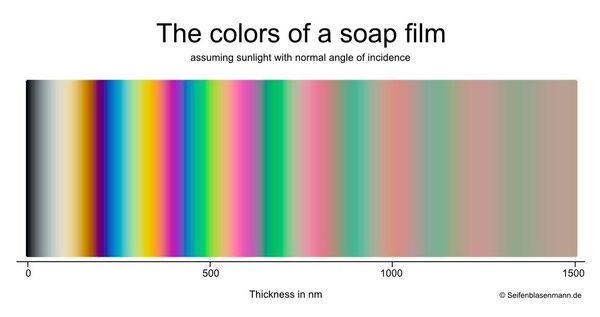
The colors of a soap film provide a precise measurement of the film’s thickness. The relationship between the colors of a soap film have been well known for more than a century. In fact, these colors provided important evidence in confirming the molecular theory of matter. The color charts on this page mapping color to thickness were prepared by a German mathematician based on well-known and uncontroversial techniques.
Bubble colors give a precise reading of a film’s thickness. One of these bubbles has much thicker walls than the other. Do you know which one?
Familiarity with the colors and the thicknesses that they represent is beneficial for soap bubble lovers and provide insights that will make you a better bubble maker and bubble juice brewer.
Soap bubbles have very thin walls. The range can be anywhere from 10 nanometers at the top of a thin-walled bubble to over 1000 nanometers. By contrast, human hair’s thickness range is on the order of 40,000 to 60,000 nanometers. Some soap films may be as few as a few soap molecules thick.
The chart below shows the colors of a soap film and the associated thickness (a vertical-oriented version of this chart is provided at the bottom of the page):
Created by Bjorn of seifenblasenmann.de and used with his permission
What causes a bubble’s colors? A soap film’s colors are the result of an interaction between the light reflected from the front and back surfaces of the soap film. The slightly different distances those two reflections travel to your eye results in a particular color. The phenomenon is known as Thin-film Interference. A bubble’s variety of colors is the result of the film, thanks to gravity, being thinner at the top than at the bottom. The colors are directly related to the film thickness (as well as light color and lighting angle). You can precisley gauge the thickness of a bubble’s walls by examining its colors.
 20:1 Dawn Pro Juice (with guar gum) |
 A thinner film created by adding extra detergent to the solution |
| In the bubbles above, the color differences between the bubbles above are solely due to the amount of detergent. Detergent concentration has a profound influence on the film thickness as discussed in Dilution. See this blog post for details. |
Become familiar with the colors associated with thin and thick films. It is valuable to be able to recognize the colors associated with thick and thin films. Take a look at the chart near the top of this page. It maps color to film thickness. The numbers on the chart indicate the film thickness in nanometers. When the film is very thin (the far left edge of the chart), it is so thin that it film reflects no light and is often called the “black film”. Against dark background, the buble will be virtually invisible. As you move to the right, you see the colors associated with increasingly thick films. Notice that yellow and blue colors only appear in films that are thinner than about 600 nanometers. Films thicker than 600 nm are dominated by increasingly dull-looking shades of green and pink. Watch the videos below to observe the progression of colors.
Due to the influence of gravity, a stable soap bubble’s walls are thinnest at the top and thickest at the bottom. The normal order of things may be temporarily disrupted if a bubble is turned or flipped by the wind or a performer, but will be restored when the bubble stops moving. When a bubble is initially blown, it may take a little while for the bubble to stabilize. You will see swirls and eddies as the bubble stabilizes.
As a bubble ages, evaporation and gravity will cause the film to become thinner and thinner causing the colors to shift. If the air is clean and the air still, you may see the bubble thin so much that it no longer reflects light and seems to disappear (the so-called “black film” stage). Watch the videos below to see this in action. The time-lapse video on the left below shows the thinning of a bubble that starts out with a relatively thick film and thins considerably. The video on the right shows a thinner-filmed bubble thinning all the way to the black film stage.
DRAFT time lapse of thick-filmed bubble as it thins Bubble Profile Test – Mr Bubbles Bubble Color Fade Demo (Solution 0620B5)
The film’s colors provide information that has practical applications. Careful observation of the colors as a bubble forms can help a bubbler determine when the film is thinning out, indicating that it is time to close a bubble. A bubble juice chef can observe the color profile bubbles and adjust the dilution to achieve a desired film thickness (since dilution influences soap film thickness).
The colors are useful in determing the effective dilution range for a particular detergent. When there is relatively little surfactant, the soap film will be very thick because the surface tension will be high. Such films will be dominated by pink and green. With increasing detergent concentration, the soap film becomes thinner producing the colors seen on the left portion of the chart. Once you reach a particular concentration called the critical micelle concentration adding more detergent has relatively little impact on the film thickness and surface tension. (JAN 2015 NOTE: There has been a question raised on SBF as to whether or not the changes in film thickness are the result of surface tension changes. There seems no question that film thickness varies with dilution but the cause may not be a change in surface tension as it has been proposed that the Critical Micelle Concentration and Critical Dilution are very different.)
The color information can be used to tune your bubble juice in the field to a particular film thickness. Thin films can be useful for maximizing size potential (in some situations), and thick films can be useful for extending bubble life.
See the article Dawn Pro Dilution Evaluation to see the color profiles that result from different dilutions of Dawn Pro dishwashing liquid.
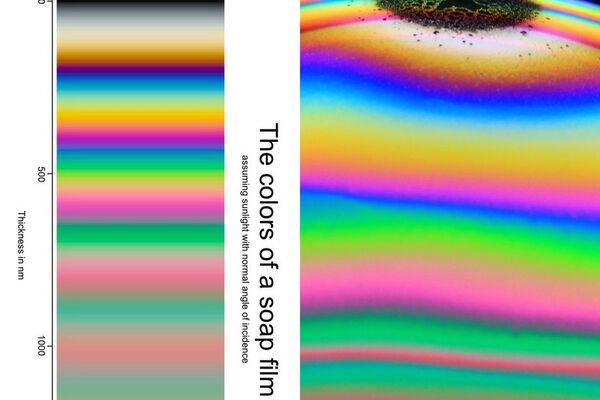
At left, Bjorn’s chart which was generated by theory. Right, a bubble dome section illustrating. Photo by Thomas Poersch. Bubble dome picture by Thommy which demonstrates the progression of colors nicely.
Dilution and Color[]
Dilution can have a big impact on bubble colors because of the way that dilution influences the soap film thickness. These color variations are especially noticeable with medium and big bubbles. They are harder to see with small bubbles. Soap dilution has some unusual properties. Read more about it in the Dilution article.
DRAFT time lapse of thick-filmed bubble as it thins
This timelapse video shows a bubble made with a pH-adjusted 40:1 mix of Dawn Pro and water as its film thins. It should help you get an idea of which colors are associated with which thicknesses.
Notice how the bubble starts with only pink and green colors. These are associated with thick-ish soap films. Yellows and belows only appear as the bubble thins.
48:1. Dawn Pro at 48:1 (water:detergent) with Edward’s longevity test setup 8:1. Dawn Pro at 8:1 (water:detergent) with Edward’s longevity test setup
Demonstration[]
2015 01 Super Giant Bubble Color and Dilution Demo-1
This video demonstrations the profound affect that dilution has on film thickness and color.
In the pictures below, pay close attention to the colors at the tops of the bubbles. These color profiles are consistent across bubble sessions as long as the lighting is similar. The difference between the 24:1 and 19:1 is fairly subtle. The difference between the 19:1 and 16:1 bubble are quite pronounced despite the much smaller difference in concentration. Changes in concentration have relatively small impacts when the concentration rises above the Critical Dilution. For Dawn Pro, the Critical Dilution seems to be somewhere between 16:1 and 18:1. The turquoise and yellow that appear at the top of the 24:1 and 19:12 bubbles are found at about the mid-line of the 16:1 bubble indicating a much thinner film for the 16:1 bubbles.
45:1. Rose and green dominate when the water:detergent ratio is high. This bubble was made with Dawn Pro at a dilution of about 45:1 (water:detergent). 24:1. The color profile of this bubble includes broad range of colors not visible in the Dawn Pro 45:1 dilution. This bubble was created with bubble juice that has a 24:1 Water:Dawn Pro ratio 19:1. HEC mix. 16:1. HEC mix. Tube with ‘Classic’ Charmy Juice similar to Dawn Pro at dilutions below 13:1 Charmy ‘Classic’ similar to Dawn Pro at dilutions below 13:1 — notice the large portion of the tube that is light amber indicating a very thin film
Very very dilute solutions create very thick films may even be colorless and glossy — as opposed to very very thin film that are colorless, glossless, and almost invisible. Very dilute solutions (where the surfactant concentration is low) have thick films that result in films dominated by shades of green and red. As the dilution becomes less extreme, the greens, reds, and pinks become more saturated, and more purples, yellows, and blues start to appear. Once the Critical Micelle Concentration (CMC), has been reached, further increases in concentration have decreasing impact on the surface tension, film thickness and color. (EDITOR: clean up confusion about CMC as CMC and critical dilution may be different).
These color changes are related to changes in the solution surface tension. Due to the way that surfactants work, these changes occur primarily in the neighborhood of the CMC.
The dilution ratios where this occurs varies (sometimes considerably) from detergent to detergent. The CMC (or at least the surface tension at a given dilution) seems to be influenced by the pH for some detergents (such as Charmy Dish Detergent ).
The images below are of similar-sized bubbles created with PEO-based bubble juice whose only difference was the soap dilution. Note how each dilution has a somewhat different color profile:
13:1 water:detergent ratio
20:1 water:detergent ratio
25:1 water:detergent ratio
50:1 water:detergent ratio
For a detailed analysis of Dawn Pro by dilution, see the article See Dawn Pro Dilution Evaluation.
More Examples[]
Note the pink/green shades typical of very dilute solutions.
See also: blog entry that compares HEC giants with dilutions from 16:1 to 24:1.
Thank you to Bjorn of Seifenblasenmann.defor the use of his chart.
Index of Refraction[]
Some question has been raised as to whether the assumptions about the relationship between color and film thickness are influenced by the components of the bubble juice. A physicist specializing in optics gave some thought to the problem and expressed confidence that the difference in the index of refraction of mixes would not vary enough to have significant impact on the relationship.
Soap Films And Molecules[]
The colors of a soap film were important evidence in establishing atomic theory of matter. The wonderful The Secret Molecular Life of Bubbles explains how the colors of a soap film were used as evidence in establishing the that matter is made up of discrete particles. It is a fascinating article that we highly recommend.
How many molecules thick is a soap film? Since molecules are not either cubes or spheres, the question cannot be precisely answered. There seems to be some consensus that you can treat soap molecules as being on the order of 4.5 nanometers thick and water molecules as being contained by a cube roughly 0.278 nanometers per side. With these estimates, we find that the amber color at the top of the color chart which indicates a film about 150 nanometers thick would be 33 soap molecules 540 water molecules thick. The “black film” (a thickness less than 25 nanometers) is less than 6 soap molecules or 90 water molecules thick. The turquoise typical of the top of a newly-formed bubble made with a 24:1 Dawn Pro dilution is about 270 nanometers thick or almost 1000 water molecules thick. You can see why on a warm dry day, it can be useful to have a thicker film.
The surfactant molecules in modern detergents may be larger than this.
More[]
A note about the color chart. If you do research, you will find a few charts which map color to thickness. There is some small disagreements between the different available charts due to slightly different assumptions about the parameters such as index of refraction, viewing angle, etc.
Dawn Pro Color and Dilution Evaluation. Click here to see an evaluation of Dawn Pro and the color profiles at different dilutions.
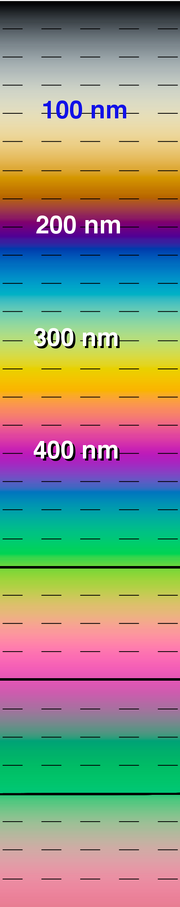
Vertical color chart. Vertically-oriented color chart with some helpful markings.
SEE ALSO[]
There are many articles and videos on the web that illustrate, explain, or discuss the physics of bubble colors. Here are a few:
- Thin Film Interference Pt. 1 – Beautiful video of a soap film thinning.
- The Colour of Soap Bubbles (illustrated physics lecture on YouTube). This lecture is a bit dry but interesting.
-
This article is a stub. You can help Soap Bubble Wiki by expanding it.